Maria L. Ganci
1Biology Department, Hobart and William Smith Colleges, Geneva, NY 14456
There is very little known about the classes of sensory neurons involved in the cold noxious response. Therefore, different crosses of Drosophila were used alongside a UAS system to determine which class(es) may be part of the circuit. The larvae from the fly crosses were tested under cold noxious stimuli and blue light to determine if the behavioral responses differed from the controls. The data from this will tell which sensory neuron is active in the circuit when the stimuli are present. The conclusion that is drawn is that the class III neurons are likely involved in the circuit.
Key words: Drosophila, larvae, noxious response, class III neuron, circuit.
The research this paper focuses on is which class of sensory neuron is part of the cold nociception response system in Drosophila. Research on the topic of cold nociception is important because there is very limited information known about the system which lends to the noxious response. Nociception is a sensory mechanism within animals which lends to the awareness of stimuli from the environment which may cause damage (Tracey, 2017). Stimuli detected by the specialized neurons will also go to the brain, and pain will be perceived in the individual (Tracey, 2017). The end result of the nociceptors picking up the stimuli is neural signals enacting nocifensive behaviors which will protect the animal (Tracey, 2017). In order to achieve nociception, TRP channels are an integral part of the circuit signaling process (Hwang et al, 2007). Their job is to mediate responses to many different kinds of stimuli (Minke and Cook, 2002).
The classes of sensory neurons that are viable to be tested are II, III and IV. Class II neurons are expressed in GMR37BO2. Class III is represented in GAL419-12 and Class IV is represented in ppk1.9. Drosophila are used because they are model organisms for a wide range of neurobiological systems. It is estimated that Drosophila and humans have up to a 75% genetic overlap, so research done on them can be applied to human experiences (Fortini et al., 2000). For this study in particular, they are optimal due to how it is possible to manipulate their genes using GAL4-UAS (Jones, 2009). Specifically, the use of GAL4 can interfere with the Drosophila neuronal system by activating or inhibiting their firing (Jones, 2009). The results of the activation or inhibition yield information about behavioral responses to stimuli (Jones, 2009). The function of each class of sensory neuron II, III, and IV will be individually knocked out via the insertion of the Kir2.1 gene into the genome of F1 larvae using the GAL4-UAS system.
Part of this experiment uses RNAi to silence genes. Gene silencing via RNAi is achieved through targeting mRNA production, thus inhibiting the translation of proteins (Sen and Blau, 2006). if knocking out a specific gene in one of the three classes of neurons prevents natural behavior to the stimulus, it would indicate that that gene, expressed by that class of neurons, is used in the behavioral response process. The end result of the nociceptors picking up the stimuli is neural signals enacting nocifensive behaviors which will protect the animal (Tracey, 2017).
The behavior of Drosophila when presented with noxious stimuli manifests in a few ways.Tracey et al. (2003) showed that general nociceptive behaviors for Drosophila larvae include rolling and contraction in response to heat. Another study done by Chattopadhyay et al. (2012) recorded a lifting of the larval head or tail in response to noxious stimuli. At the end of this study, it is expected to be understood which sensory neurons play a role in the cold nociception circuit. The overall hypothesis is that either sensory neuron class II, III, or IV are involved in the cold nociception neural circuit.
MATERIALS AND METHODS
Organisms
The flies came from online stock sources that were purchased for use. The stocks purchased were ppk1.9 (32078), Kir2.1 (6596), UAS-RNAi (31289), GAL419-12, and GMR37BO2. The GAL419-12 and GMR37BO2 were a gift of Dan Cox. All of the flies were incubated at 25 degrees Celsius. Virgin female flies were collected using a CO2 anesthetization method. Experiments were conducted on their third instar larvae.
Cross System Process
A custom-built fluorescent microscope was used to test the crossing process. Fluorescent light was shone on third instar larvae. The goal was to visualize the GFP (green fluorescence protein) that was crossed with the fly.
Kir2.1
Ppk1.9, GAL419-12 and GMR37BO2 adult flies were crossed with UAS-Kir2.1 flies. Not enough females from the GAL419-12 cross were collected for their larvae to be used. Third instar larvae were collected from the incubated vials and placed onto damp room temperature Peltier plates. A Celestron video microscope camera was used to analyze the larvae. Using Photobooth and Image-J, the original length of larva before stimulus was recorded. Then, the Peltier plates were cooled until the thermometer read under 61 degrees. Once the cold stimulus was applied for 30 seconds, another picture was taken of the larvae. The process was repeated with control larvae without the Kir2.1 gene. A chi-square test was done to analyze the data.
Optogenetics
During a week-long incubation period, virgin females were collected from the ppk1.9, GAL419-12, and GMR37BO2 vials after being crossed with Ch2R flies (36495). Their larvae were collected, and a picture was taken before stimulus using the Image-J and Photobooth software. A photo was taken under 470 nm of blue light. An after picture was taken to using the same software after the light was applied for 5 seconds. The rhodopsin creates retinol which will eventually depolarize the neuron. This was done in attempt to trigger cold behaviors in the absence of a cold stimulus, using light to determine which class of sensory neurons are involved in cold nociception. The process was repeated with control larvae without the Ch2R gene. A two-sample t-test was conducted to analyze the data.
RNAi
8 adult female flies from the ppk1.9 stock (stock 31289) were crossed with 3 male RNAi-UAS flies. The same methods from the Kir 2.1 experiment were applied to this experiment for the control and experimental larvae. A chi-square test was done to analyze the data.
RESULTS
As seen in Table 1, testing the Kir2.1 larvae for behavioral responses using cold suggested that there was no significant difference in the number of behaviors between control larvae and experimental larvae (Chi square, x=8.483, 6 df, p>0.05; Table 1). Figure 2 focuses on reporting results from optogenetic testing. The GAL419-12 control, and GAL419-12 experimental tests show statistically significant differences in terms of larval body contractions. That result is seen in how the p value for experimental GAL419-12 is less than 0.05, and the p value for the control GAL419-12 is greater than 0.05 (Two sample t -test, t=4.41, 23 df, p<0.05); (Two sample t-test, t=0.131, 13 df, p>0.05; Figure 1). Still in Figure 2, GMR37BO2 control and experimental both had outcomes with p values greater than 0.05 therefore the differences are insignificant (Two sample t-test, t= 1.31, 29 df, p>0.05); (Two sample t-test, t= 1.59, 45 df, p>0.05; Figure 1). The data in Table 2 shows that for the GMR37BO2 flies crossed with the UAS-RNAi flies, the differences in contraction before and after light exposure was insignificant.
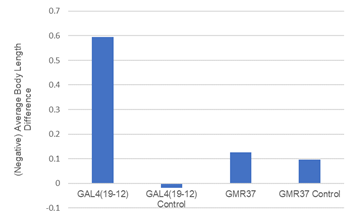
Figure 1. Comparing experimental and control larvae strains’ contraction responses when presented with 470nm of blue light. (Two sample t -test, t=4.41, 23 df, p<0.05); (Two sample t-test, t=0.131, 13 df, p>0.05); (Two sample t-test, t= 1.31, 29 df, p>0.05); (Two sample t-test, t= 1.59, 45 df, p>0.05).
| Larvae type | CT | HTL | Roll | No Behavior |
| Ppk1.7 | 3 | 14 | 1 | 10 |
| GMR37B02 | 8 | 7 | 4 | 16 |
| Controls | 11 | 15 | 5 | 28 |
Table 1. Comparing experimental and control larvae strains’ contraction responses when presented with 470nm of blue light. (Two sample t -test, t=4.41, 23 df, p<0.05); (Two sample t-test, t=0.131, 13 df, p>0.05); (Two sample t-test, t= 1.31, 29 df, p>0.05); (Two sample t-test, t= 1.59, 45 df, p>0.05).
| Behavior | No behavior | |
| Experimental | 1 | 6 |
| Control | 19 | 40 |
Table 2. Experimental larval behavioral responses to noxious cold with UAS-RNAi x ppk1.9 (stock number 31289). (Chi square, x=0.951, 1 df, p>0.05).
DISCUSSION
The data collected points to a series of possible conclusions. Kir2.1 prevents neurons from depolarizing which silences individual classes of neurons. Thus, if the correct sensory neuron is silenced, then there should be no behavior. Based on the results in Table 1, the differences between experimental larval behaviors to cold and the control behaviors to cold are insignificant. This means that the experimental larvae were showing their natural behavior. The insignificance insinuates that the neurons that the UAS-Kir2.1 knocked out were not part of the cold behavioral response circuit. Furthermore, if the class II (GMR37BO2) and class IV (ppk1.9) neurons were part of the cold nociception response circuit, they would show a decrease in cold nociceptive behavior compared to the controls. Instead, they showed statistically similar behaviors, which leads to the conclusion that they are likely not part of the circuit.
The three fly stocks were crossed with adults from the UAS-Ch2R (Channel 2 Rhodopsin) strain to activate the nociceptive responses to light instead of cold. When light hits the rhodopsin, it creates retinol, which affects the chain that will depolarize the neuron. If the light had elicited cold nociceptive behaviors, then it is implied that the same behavior would have been produced from cold noxious stimuli. This data signifies which of the sensory neurons are involved in the circuit. The data in Table 2 shows statistical significance in differences between behavioral contraction in the control and experimental GAL419-12 larvae when presented with blue light. Thus, the data shows that the sensory neuron III which is expressed by the GAL419-12 could be active in the response. However, for the GMR37BO2, the opposite was true: the control and experimental larvae showed no significant differences when hit with the blue light which means that they are likely not part of the response.
For the RNAi silencing test, the class of neurons expressed in the ppk1.9 fly stock was class II. RNAi prevents the mRNA gene from forming. This is important and done because if the behaviors persist in the absence of that gene, then the gene is not important in the noxious cold response. The genotype of the RNAi flies shows that there is UAS bound to SYT1 (Bloomington). SYT1 is Synaptotagmin (Flybase). Research shows that synaptotagmin is a membrane protein. When the neuron is activated and Ca2 influx begins, the protein works so the neurotransmitter filled vesicle can bind to the presynaptic terminal (Littleton et al., 1994). Therefore, with the UAS-RNAi system knocking out the SYT1 gene in class II sensory neurons, no response behavior to cold can ensue. When testing larvae with RNAi interference, the chi square test showed he differences between control and experimental behaviors were found to be statistically insignificant. However, the data suggests that the gene might be important in the circuit due to how the data, while statistically insignificant, could be found to be significant if the sample size was larger as the values are fairly different. Given these conclusions, it newly understood that either class III sensory neurons as expressed by GAL419-12 or class IV sensory neurons as
REFERENCES
Chattopadhyay A, Gilstrap A, Galko M (2012) Local and global methods of assessing thermal nociception in Drosophila larvae, Journal of Visualized Experiments 63:3837.
Fortini M, Skupski M, Boguski M, Hariharan I (2000) A survey of human disease gene counterparts in the Drosophila genome, Journal of Cell Biology 150:F23-FF30.
Hwang R, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey D (2007) Nociceptive neurons protect Drosophila larvae from parasitoid wasps, Current Biology 17:2105-2116.
Jones W (2009) The expanding reach of the GAL4/UAS system into the behavioral neurobiology of Drosophila, Korean Society for Biochemistry and Molecular Biology 42:705-712.
Littleton, J.T., Stern, M., Perin, M., Bellen, H.J. (1994). Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc. Natl. Acad. Sci. U.S.A. (23): 10888—10892.
Minke B, Cook B (2002) TRP channel proteins and signal transduction, American Physiological Society 82:429-472.
Sen T, Blau H (2006) A brief history of RNAi: the silence of the genes, The FASEB Journal 20:1293-1299.
Tracey D (2017) Nociception, Current Biology 27:R129-R133.
Tracey D, Wilson R, Laurent G, Benzer S (2003) Painless, a drosophila gene essential for nociception, Cell Press 113:261-273.
Leave a comment